Recently, the prestige journal Advanced Functional Materials published online the latest research results entitled Triple Enzyme-Regulated Molecular Hydrogels for Carrier-Free Delivery of Lonidamine by the team of Professor Wenying Zhong from China Pharmaceutical University. Professor Wenying Zhong and associate research professor Keming Xu are the co-corresponding authors of this article. The PhD student of class 2018 Can Wu is the first author, and China Pharmaceutical University is the sole corresponding organization of this article. The paper could be read online: https://doi.org/10.1002/adfm.202104418.
There are a variety of highly expressed enzymes in the tumor microenvironment, and these enzymes are essential for the occurrence, development and metastasis of tumors. Because enzymes exist in multiple organelles in cancerous cells, they are considered to be undruggable by small molecule drugs. In recent years, the use of enzymes to regulate supramolecular self-assembly and to construct drug-carrying hydrogel systems becomes one of the new strategies in tumor diagnosis and treatment. Among them, enzyme-instructed self-assembly utilizes the alkaline phosphatase or other highly expressing enzymes to trigger the self-assembly of small peptide molecules and formation of nanofibers for selectively killing of cancerous cells. Since self-assembled peptide nanofibers show excellent targeting function and biocompatibility, they have attracted much attention in tumor diagnosis and treatment.
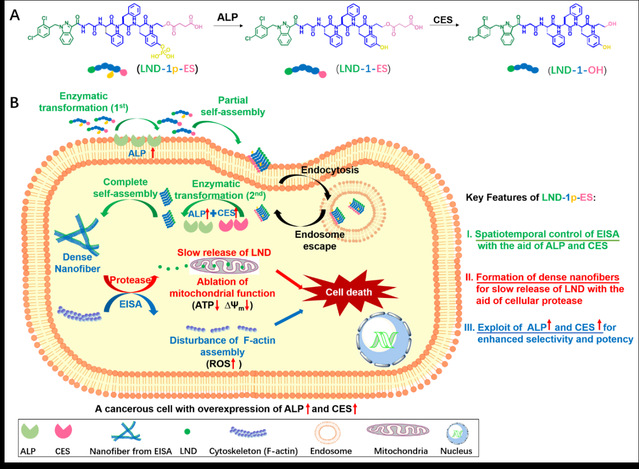
In view of this, the Zhong team designed a triple enzyme-responsive self-assembling peptide derivative LND-1p-ES for carrier-free delivery of lonidamine (denoted as LND hereafter). The structure of LND-1p-ES consists of: (i) LND as the capping group that can not only enhances the self-assembling capability of peptides, but also releases the pure drug LND slowly under the action of intracellular proteases; (ii) a Gly-Phe-Phe-Tyr sequence, used to enhance the self-assembling capability of the peptide derivative; (iii) a phosphorylated tyrosine part, as the substrate of alkaline phosphatase (ALP); (iv) a succinate monoester part, as the substrate of esterase (CES). Since ALP is located in the cell membrane and cytoplasm, and CES is mainly located in the cytoplasm, the high expression of ALP and CES in cancerous cells enables the spatiotemporal control of the intracellular self-assembly of LND-1p-ES. The ALP on the cell membrane first catalyzes the conversion of LND-1p-ES to LND-1-ES, triggering the primary self-assembly. After that, LND-1p-ES/LND-1-ES enters the cytoplasm and is converted into hydrogelator LND-1-OH under the combined action of intracellular ALP and CES. The latter forms a dense nanofiber network in the cell, that not only interferes with the assembly of actin and increase the ROS levels in cancerous cells, but also serves as a drug reservoir for slow release of LND under the action of intracellular proteases, thereby ablating mitochondrial function and inducing cell death.
The main innovation of this research lies in the design of LND-1p-ES that integrates the drug and the carrier in a single molecule, which exploits three enzymes to precisely regulate the self-assembling process. Comparing to the pure drug (LND), or single/none enzyme responsive hydrogels, the LND-1p-ES hydrogel treatment demonstrates significant anti-cancer efficacy in vivo and in vitro. In addition, LND-1p-ES hydrogel is less irritating at the injection site, and shows satisfactory biocompatibility in animal studies. This study utilizes a variety of enzymes that are highly expressed in cancerous cells to enhance the anti-cancer efficacy of lonidamine, which provides a general strategy for the construction of peptide-based nanomaterials for drug delivery applications. In future, other signaling molecules existing in the disease/lesion sites can be used to regulate the self-assembling behavior of peptide-drug conjugates, which shows great promise in the development of disease-targeted functional soft materials.
This research is funded by the National Natural Science Foundation of China (No. 51973234, 21775165), Basic Research Program of Jiangsu Province (No. BK20191326) and a Double First-Class Subject Project of China Pharmaceutical University (No. CPU2018GY25).